AI matches radiologists in detecting prostate cancer in NHS-backed multi-centre study
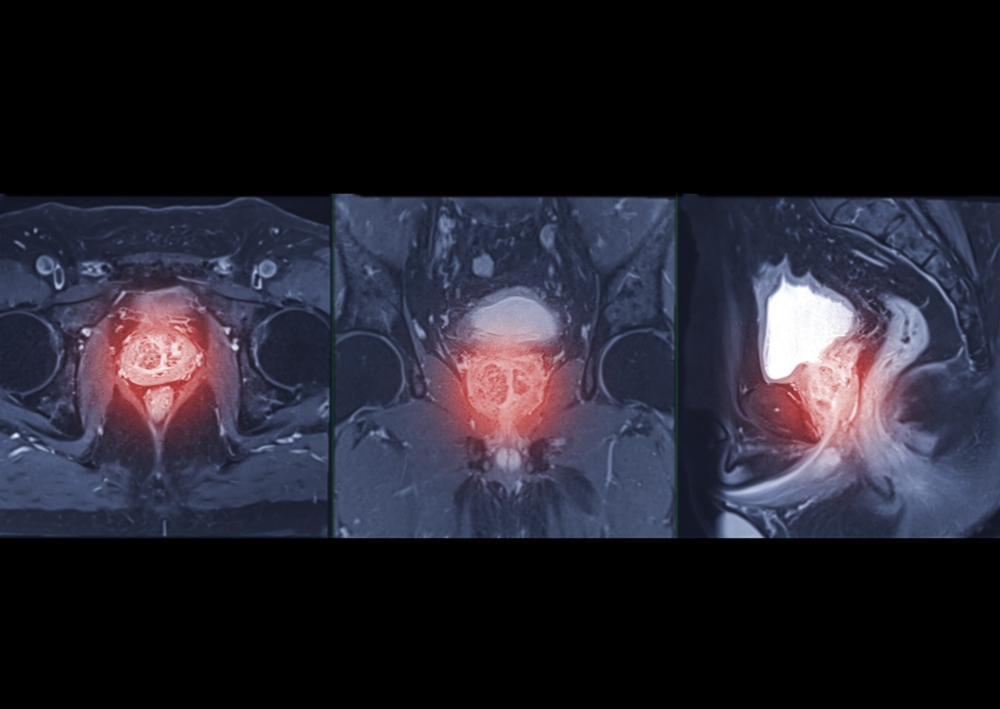
A trial of Pi AI software, already in use in the NHS, has shown high accuracy in analysing MRI scans to distinguish clinically significant prostate cancer.
Hampshire Hospitals NHS Foundation Trust and Lucida Medical have announced the results of a five-year collaboration. Results from the PAIR-1 (Prostate AI Research – 1) study shows that the Pi AI software, now in use in NHS and European hospitals, performs as well as expert radiologists at detecting prostate cancer from magnetic resonance imagining (MRI) scans. Prostate cancer is the most common men’s cancer, leading to around 12,000 deaths in the UK every year.
PAIR-1 is a collaborative research study between eight NHS Trusts and Lucida Medical, approved by the NHS Health Research Authority and funded by the company. The study partners gathered historical data from over 2,000 patients and used this to develop, train and validate Pi, a software platform that uses artificial intelligence (AI) algorithms to analyse magnetic resonance imaging (MRI) scans to help distinguish clinically significant prostate cancer.
Dr Antony Rix, CEO and Co-Founder at Lucida Medical, highlights that “every year, over 50,000 men in the UK and 1.5 million men worldwide are diagnosed with prostate cancer. The disease may start slowly, but can be deadly if it’s not caught early, killing 12,000 men in the UK and 400,000 men around the world each year.”
An MRI scan is a key step to diagnose prostate cancer. The MRI is used to help identify patients at low risk who can avoid a painful, invasive biopsy, and to locate possible lesions so that higher-risk patients can have a targeted biopsy to maximise the chance of finding cancers that need treatment. Mark Hinton, CTO at Lucida Medical, explained: “Pi is medical device software that is CE approved for use in clinics. We developed Pi to automate key steps like outlining lesions and calculating risk scores, to assist radiologists to make these challenging decisions.”
Dr Francesco Giganti, Associate Professor of Radiology at University College London, presents the results of the PAIR-1 study today at the European Congress of Radiology (ECR) in Vienna. He noted that “this research found that Pi is non-inferior to multidisciplinary team-supported radiologists across a validation set of sequential cases from 6 NHS hospitals with a wide range of MRI scanner types. This is the first time that a commercial AI for prostate MRI has been tested on diverse, real-world data.”
Dr Aarti Shah, Consultant Radiologist at Hampshire Hospitals NHS Foundation Trust, was Chief Investigator on the study. She highlighted that “analysing MRI scans is a time-consuming task for expert radiologists, and there are too few of us in the UK and many other countries. Pi offers exciting potential as an aid to help reporting radiologists in triaging workloads as well as producing visual reports to aid contouring of lesions for biopsy.”
“We founded Lucida Medical with a shared vision to use AI to transform the diagnosis of cancer. Five years on, it is wonderful to see this working in practice and recognised by a major journal and conference,” added Prof Evis Sala, Co-Founder of Lucida Medical, Professor of Radiology at the Università Cattolica del Sacro Cuore and Chair of Department of Diagnostic Imaging and Radiotherapy at the Policlinico Universitario A. Gemelli, IRCCS in Rome.
Pi is available for use in the UK and Europe to support the diagnosis of prostate cancer.
At ECR 2025, Dr Giganti’s presentation, AI-powered prostate cancer detection: a multi-centre, multi-scanner validation study, took place in session CTiR 16 – Clinical Trials in Radiology: spotlight, in Room N on Feb 28 at 16.00 CET. The research is also published in European Radiology at https://doi.org/10.1007/s00330-024-11323-0.